Antibiotic residue tests: What they are and how to better comply with Brazilian legislation
What are false positives, false negatives and false violators?
Undoubtedly, the concern with the presence of contaminating substances that are not part of the composition of milk is present in the daily lives of producers of this food.
Therefore, the milk production chain is constantly monitoring residues of veterinary drugs, in order to ensure food safety for consumers.
Previously, we talked in other blog articles about the presence of additives and substances that increased the volume or durability of milk. In today's agenda, we'll talk about the different types of existing methods, their detection fundamentals, and we'll get into a subject that can cause a lot of controversy: antibiotic residues and the interpretation of their results. To know:
- Positives;
- Negatives;
- False positives;
- False negatives;
- False-violative.
Bem, a presença de resíduos de medicamentos, como antiparasitários e antimicrobianos (antibióticos), pode ser comum no leite produzido, uma vez que estes medicamentos têm seus usos permitidos.
However, the amounts must not exceed the pre-established values in Brazilian legislation. These limits are called Maximum Residue Limits (MRL in Portuguese, or “MRL - Maximum Residues Level” in English).
Thus, to monitor these values, rapid screening tests are initially performed to track these substances and understand whether they are present in the milk. These tests are called qualitative, where their presence or absence is determined.
In this way, we will review the existing analysis methods and a chronology of their evolution.
Residue monitoring and false violations
Well, so far we have seen that the Brazilian legislation has its parameters and levels of acceptance for these antibiotic residues, but how is this monitoring carried out?
Monitoring methods are classified into two types, screening and confirmation. That is, it is done in two stages.
In the first, there is screening through qualitative or semi-quantitative tests. These can be of the following types:
- Microbial or microbiological inhibition
- Immunological
- Based on chemoreceptors
Microbiological growth inhibition halo method
The first method used was the inhibition of microbial growth, which was based on the principle of inhibiting the growth of bacteria in a petri dish, previously sown with a known amount of bacteria, to which a volume of milk was added and if there was the presence of antibiotic residues above a certain concentration, there would be inhibition of the growth of bacteria, when measuring the size of the inhibition, it would be known if the concentration of antimicrobials is above the maximum limit allowed.
The result of this method takes from 12 to 24 hours to be ready, which makes its use in analyzes of receiving and releasing milk for unloading unfeasible. These methods were used in the food industry until the beginning of the 1970 decade, and do not identify the type of antimicrobial and the response depends on the sensitivity of the bacteria used to the antimicrobial present in the sample. In addition, they are unable to identify the type of antibiotic present in the sample and the result is presence or absence, without differentiating the antimicrobial family.
Elisa methods on microplates
With the evolution of immunochemistry at the end of the 1980 decade, the elisa technique appeared -“enzyme-linked immunosorbent assay”- analysis by an enzyme linked to an immune substrate- methods were created that could identify a specific substance, in a way effective, each elisa analysis specifically identified a family of antimicrobial and without interference from other compounds, this type of analysis meant a huge qualitative leap, as it was now possible to know exactly which antimicrobial was present in the milk, without interference from other contaminants. Despite the analysis time being shorter than that of the microbiological method (around two hours), the major problems with this technique were the difficulty in handling the microplates, the number of steps in the process (5 steps), operational problems and analytical errors, in addition to the higher cost. This type of method was practically restricted to research centers and universities.
Lateral flow immunochromatography methods
Este tipo de método foi introduzido no mercado mundial no início da década de 1990, e começou a ser utilizado no Brasil, introduzido pelo diretor técnico da Somaticell, quando este desempenhava as funções de gerente da qualidade de um grande laticínio em São Paulo, em 1994.
In this scenario, there is a category of tests that identifies the presence of these residues without quantifying values. So, even if these are below the maximum limit of the legislation, they can point to positive results.
In these cases, even with a small presence of antibiotic residues, the milk can be considered fit for consumption, as it respects the limits of the legislation.
We have separated the theme into the following topics to facilitate the reading and understanding of the whole scenario:
- The antibiotic therapy
- Why are antibiotic residues present?
- A legislação Brasileira sobre o uso de antibióticos
- Waste monitoring
- Precautions when performing the tests
- Qualitative methods for detecting antimicrobial residues
- False-violatives and the GAP of qualitative tests
- Quantitative tests
The antibiotic therapy
Antibiotics are widely used in cattle for the treatment of various infectious diseases, such as mastitis. These chemical substances have the role of killing or inhibiting the growth of microorganisms that cause pathologies in humans or animals.
Thus, when detecting the presence of these microorganisms in the herd, the veterinarian can use the most diverse range of antibiotics available for cattle, depending on the type of bacteria or parasite that affects the animal.
The use of antibiotics is a very efficient weapon for the control and eradication of diseases, when used properly and under prescription. When treatment is done irresponsibly, without veterinary monitoring, it can trigger several public health problems.
Among them, the most famous is antimicrobial resistance. According to the WHO (World Health Organization), this is one of the biggest public health challenges in the world. Therefore, veterinary professionals are responsible for controlling the treatment of animals and testing for antibiotic residues in milk to further increase the safety and quality of the raw material.
In this scenario, antibiotic residue tests are control methods used to identify the presence of residues. In this way, it is possible to have greater control over the presence of these substances that interfere with the quality of the milk produced.
The main types of tests available on the market can be divided into rapid tests or screening tests, which bring qualitative results (positive or negative), and confirmatory tests, which involve a longer process.
Main classes of antimicrobials
Among the most used antibiotics in cattle, we can mention the following classes as the most used in herds:
- Beta-lactams: Penicillin, Ampicillin, Amoxicillin, Cloxacillin, Ceftiofur and other Cephalosporins;
- Tetracyclines: Tetracyclines, Oxytetracyclines and Chlortetracyclines;
- Aminoglycosides: Streptomycin, Neomycin, Gentamicin;
- Macrolides: Erythromycin, Tylosin, Tilmicosin;
- Sulfonamides and Trimethoprine: Sulfamethazine, Sulfadimethoxine, Sulfathiazole and more than 15 other compounds;
- Quinolones: Enrofloxacin, Norfloxacin, Ciprofloxacin, Ofloxacin, Rosoxacin, Sparfloxacin, Floroxacin, Pefloxacin, Aminofloxacin, Lomefloxacin, and more compounds.
- Amphenicols: Chloramphenicol, Florfenicol, Thiamphenicol;
But attention! Even if the classes are the same, do not use antibiotics suitable for human medicine in cattle. There are still no studies on the discard period. Then, follow the instructions of your veterinarian on your property.
Why does antibiotic residue form?
For the treatment of any bacterial or parasitic infections that use antimicrobials, regardless of the route of administration, there may be residues of these drugs in milk, even in small amounts.
The most common routes of administration in cattle are intramammary, intrauterine, oral, muscular or through the skin. Antibiotics are absorbed into the bloodstream after application and can then pass into the milk.
Typically, the presence of antibiotics is associated with the following conditions:
- Extra-packaged, inappropriate or illegal use;
- Lack of employee training;
- Lack of records of treated cows;
- Treatment failures;
- Failures in posology, dose and form of administration;
- Dilution of antibiotics in milk;
- Management failures;
- Failure to respect the grace period;
- Number of offspring, the more, the lower the metabolism;
- Milk production, the lower the productivity, the higher the residue concentration in the milk;
- Do not discard milk from treated cows.
Brazilian legislation on antibiotic residues and false violatives
A legislação brasileira é bastante cuidadosa no quesito de monitoramento dos produtos de fonte Agrícola e da Pecuária. Dessa forma, o Plano Nacional de Controle de Resíduos em Produtos de Origem Animal (PNCRC) estabelece para leite os Limites Máximos de Resíduos (LMR).
Os parâmetros de LMR são descritos no Decreto 9013 de 2017. Neste documento Normativo está descrito o Subprograma de Monitoramento, de Investigação e Exploratório, para execução do PNCRC.
According to Decree 9013, in its article 248, normal milk is that which does not contain residues of products for veterinary use and contaminants above the maximum limits established. In addition, this decree also describes the Sampling Plan and the procedures for collecting, preparing, packaging and sending samples for analysis.
For current control, every year the Ministry of Agriculture, Livestock and Food Supply (Mapa) publishes the scope of analytes that need monitoring, as well as the number of samples that the parent company needs to collect.
Precautions when performing the tests
The first mistake that can occur when carrying out a qualitative test is to do the analysis right after milking. This can generate false positives, due to natural inhibitors that are present and active in milk for a period of up to 3 hours.
So, to circumvent this occurrence, the sample must be heated to 80 °C for 5 minutes and then refrigerated. This inactivates the natural inhibitors and prevents a false positive result.
But as we mentioned earlier, these tests usually take place hours later, already in the tank of trucks, arriving at the dairy industry.
Ways of detecting CMP
We have seen so far that it is of paramount importance to be careful with refrigeration during the transport period of the milk sample, in view of the presence of psychotrophic microorganisms.
It is necessary to have this prior control, before seeking the reasons for the increase in CMP in milk, whether due to bacterial growth or the addition of whey.
Tendo os cuidados prévios, a melhor forma de detecção das quantidades CMP (caseinomacropeptídeo) é através do método HPLC, sigla em inglês para cromatografia líquida de alto desempenho.
Through this analysis, it is possible to perceive the quality of the milk and evaluate its ability to convert it into derivative products such as cheese and yogurt, which are dependent on intact casein.
Qualitative and semi-quantitative methods for detecting antimicrobial residues
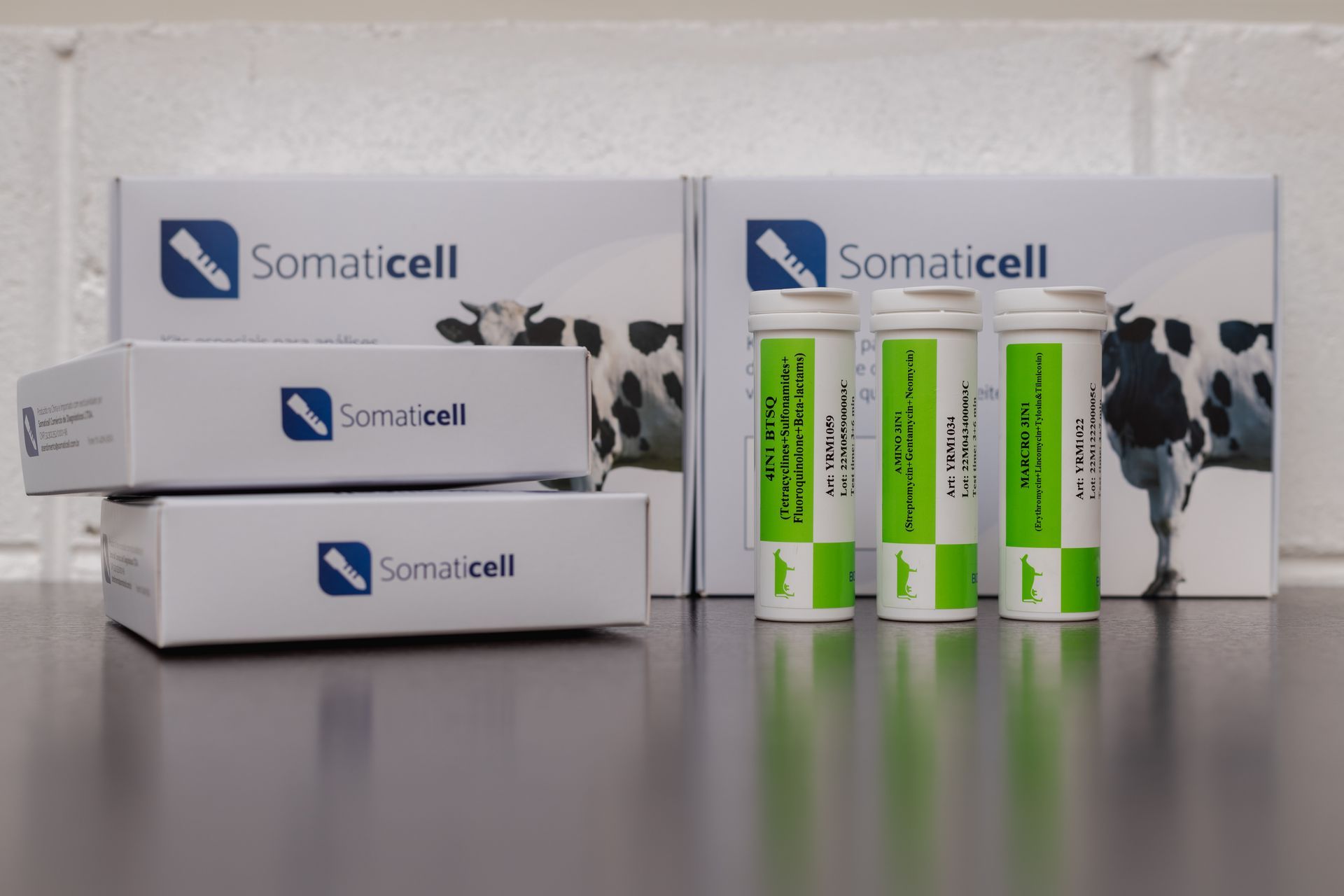
In Brazil, there are several antimicrobial residue detection kits. However, it is necessary to select the screening tests that are most suitable for your situation, according to the limits of detection of antimicrobial residues.
At the Brazil, The Somaticell Diagnósticos works with kits designed to serve the Brazilian market, with the greatest protections in terms of drug detection capacity, coverage of detected families, with 100% of the needs described in the PNCRC – National Waste and Contaminant Control Plan – of the MAPA, to ensure that dairies receive milk with residues below legislation, but without discarding good milk as contaminated milk.
Com os Kits 2 em 1 da Somaticell, é possível realizar o monitoramento adicional previsto na IN76 e na IN77, com coberturas de 90% ou 95%, ou 100% das drogas antimicrobianas comercializadas para vacas leiteiras.
The analyzes carried out to identify Beta-lactams and Tetracyclines manage to reach an efficiency of 100%. So, according to the customer's needs, the kits may be suitable for carrying out the diagnosis in the herd.
Regarding the methodology, the kits Somaticell Diagnoses are based on Elisa (Enzyme Linked Immunosorbent Assay), manufactured under the strictest quality standards and meeting the detection limit requirements required by MAPA – Ministry of Agriculture- ANVISA – National Health Surveillance Agency - and Codex Alimentarius.
In this way, Somaticell diagnostics can, through these kits, identify Beta-Lactams, Tetracyclines, Sulfonamides and Fluorquinolones, Aminoglycosides, Macrolides and Amphenicois. Somaticell markets combined or individual solutions for each of the antibiotic families sold in Brazil.
Interpretation of results using qualitative and semi-quantitative tests
Qualitative tests for the detection of antibiotic residues can identify the presence of these substances even in amounts lower than that established by the MRL.
What happens when residue is detected by these screening tests? Means that the test detected a value of antibiotic residues at the limit of detection of the method, which can be close to the MRL (Maximum Residue Limit), or below the MRL.
Using only these tools, the milk could automatically go to disposal, right?
The protocol for disposal of milk unfit for human consumption must be proven by the unequivocal certainty that the concentration in the product is in fact above the legislation. For these cases, some countries establish an official procedure to assist industries in the application of this protocol, which unfortunately is not the case in Brazil.
For greater clarity of the subject, we will use the FIL-IDF definitions on the subject:
FALSE POSITIVES:
- Definition: the method gives the result as positive, but the analyte – target substance – is not present;
- In this case, we have a false positive result, as there is no residue in the analyzed sample;
- Main causes:
- Abnormal milk – sour milk;
- High concentration of solids in the sample, eg. concentrated whey;
- High somatic cells;
- High fat.
FALSE NEGATIVES
- Definition: the method gives the result as negative, but the analyte – target substance – is present and above the norm;
- When a method states that it detects active principles, but the negative result does not mean that the residues present above the norm (Brazilian legislation) are absent;
- Main causes:
- Method poorly dimensioned for the purpose
- Example 1: microbiological method that aims to detect multiple drugs is validated only for beta plus tetra and will give the false impression of safe milk, even with the drug present in the milk above the LMR.
- Example 2: method for beta lactams that detects cephalexin at 500ppb, while the norm is 100 ppb, that is, a negative result does not guarantee compliance with the norm.
FALSE VIOLATIONS
- Definition: The test detects antibiotic residue, the residue is indeed present, the result is classified as positive, but the level of antibiotic residue is below Brazilian legislation;
- Cause: This is the typical case of the detection capacity below the MRL, which is due to the reaction capacity between the antibody/enzyme and which depends on the cross reactions between the antibody and the antibiotic and the chemical stereo interferences between the different molecules of the antibiotics and the receptors (antibody/enzyme), which can couple differently and thus cause non-linear detections between the different components of each family;
- Exemplo: No kit
2in1 Beta+ Cefalexina+ Teracilcinas temos a detecção de Penicilina a 2 ppb (50% do LMR) e do Ceftiofur a 80ppb (80% do LMR), demonstrando a ocorrência de reações cruzadas diferentes entre as diversas moléculas e o anticorpo.
Quantitative tests
For an exact and precise determination of the concentration of veterinary drug residues, the quantitative methodology indicated in Brazil and in other countries is HPLC MS/MS (high performance liquid chromatography with mass/mass detector), which manages to quantify the amounts at the level of ppbs (part per billion or mg/l for example). It turns out that this type of equipment is difficult to operate, as it requires highly specialized technicians, high investment, low capacity for the number of analyzes and long response time, meaning that in Brazil, for example, we have few centers able to carry out this type of analysis. analyses, located in regions far from the large dairy basins.
As a result, we have an average time to carry out a quantitative sample of around 15 days, from collection to result. In practice this means that this type of analysis only serves as a fiscal analysis, or for government programs and academic studies.
But how to resolve the issue of condemning milk? With the methods of Somaticell, it is possible, through the application of violation analysis methods, knowing the drug involved in the violation, some aspects of the characterization of the sample analyzed and the method applied, to be able to estimate with a high degree of certainty whether the sample is above Brazilian legislation and whether the milk should be condemned, for violating it.
In this way, you who are a Somaticell customer will be able to take advantage of this exclusive service to apply a milk condemnation sanction, or not, after applying the specific method.
What can we conclude about false violatives?
When we talk about antibiotics, one thing that should be clear is that drugs have not yet been made that the body of cattle can fully absorb, so the residues. Therefore, whenever these drugs are administered, it is possible to find traces of their elimination in milk.
So, based on this principle, there is a legislative tolerance, which allows a Maximum Residue Limit (MRL).
Within this universe, we have the issue of false-violatives, which have a certain amount of antimicrobials, however, they are below the MRL, and therefore suitable for consumption.
Well, if the legislation provides that it is possible to maintain food safety even in the presence of these antibiotics, then less milk is discarded.
But in order to avoid these discards, it is necessary that the producers consciously use these antimicrobials, respect the disposal time and always remember that the established maximum residue limit is applied for each animal and the analysis in collection tanks or trucks is a mere liberality of the sanitary authority, to establish an operationally feasible control point.
Para a detecção de resíduos de todos os antibióticos vendidos no Brasil e permitidos para uso pelo MAPA, conte com a Somaticell para o fornecimento dos kits de detecção. Nós temos uma cobertura completa das demandas técnicas e com o suporte técnico para ajudar você a aplicar a legislação da melhor forma possível e com a melhor relação benefício/custo.
Para saber mais informações sobre nossos serviços, entre em contato com a gente e fale com um de nossos especialistas!

Conheça nosso App

Our Educational Videos
Somaticell on Social Networks